Introduction
In 2021, India was ranked 85th out of 180 countries on the Corruption Perception Index. Corruption acts like a parasite for the country and causes huge damage to the society. Science could help in reducing it by increasing the conviction rates of corrupt officials in the country.
To curtail it, in a recent judgement, the Supreme Court itself has asked the investigating agencies to adopt scientific methods in crime detection to save the judicial system from low conviction rates.
A bench composed of Justice K. S. Radhakrishnan and Justice A. K. Sikri said that, “The criminal judicial system in this country is at a crossroads,”. Moreover, “Reliable, trustworthy, credible witnesses to the crime seldom come forward to depose before the court and even hardened criminals get away from the clutches of the law. Even reliable witnesses for the prosecution turn hostile due to intimidation, fear and a host of other reasons. Investigating agency has, therefore, to look for other ways and means to improve the quality of investigation, which can only be through the collection of scientific evidence”.
What is the Phenolphthalein test?
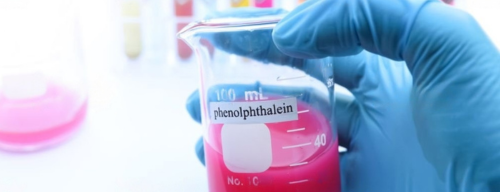
Phenolphthalein powder, is a white-coloured powder, which comes under the family of tracing chemicals or Detective Dyes, as they are used by law enforcement agencies to collect evidence against the accused individuals. It is applied on the currency notes to confirm the exchange of notes. The two essentials of bribery are: i) a communication of demand for cash or kind, and ii) the acceptance of such a demand. This transaction can be proven by the Phenolphthalein powder. It acts as a chemical indicator since it changes colour in an alkaline solution. Although there are many tracing chemicals used by the agencies, phenolphthalein powder and anthracene powder are specifically used by anti-corruption agencies, to trace the exchange of currency between two individuals, as it signifies the acceptance of the currency.
The test works on Locard’s principle, derived by Dr. Edomond Locard. The principle states that “Every Contact Leaves a trace”; which simply means that whatever is touched by the culprit will act as evidence against him and will be known as silent evidence. Unlike human evidence which could be manipulated, this could not be manipulated as this is factual evidence, later confirmed by an expert in the field.
In the case of Raghbir Singh v. State of Punjab[1], a Passenger and Goods Tax Clerk II, was accused of taking 50 rupees bribe from a truck owner. During the trial two different stories from each side were presented. The plaintiffs argued that the currency notes were recovered from the accused, however, the accused claimed the opposite. As the currency notes were not laced with the Phenolphthalein powder, the Counsel for the investigative agency was not able to prove the accused guilty beyond reasonable doubt. Hearing the appeal, Hon’ble Justice P.N. Bhagwati and Justice R.S Sarkaria of the Supreme Court of India established the importance of the Phenolphthalein powder method by establishing that while catching a public servant in the act of bribery, it is desirable that the currency notes, which are used for trapping, should be treated with the phenolphthalein powder so that the handling of such notes can be detected by a chemical process. This ensures that the courts do not have to be dependent on just the oral evidence, which can sometimes be of dubious nature. As the world enters a technological age, it becomes imperative that science-oriented methods be adopted on a large scale for the detection of crimes. However, it must be noted that in the case ofKilli Ram vs State of Rajasthan, the Supreme Court has also held that it is not mandatory to conduct the Phenolphthalein test.
Process of the Phenolphthalein Test
According to the Working Procedure Manual: Chemistry, of the Directorate of Forensic Science, Ministry of Home Affairs, Government of India, when a complaint is filed by a person at the anti-corruption bureau, the bureau forms a team and lays a trap to catch the culprit red-handed with concrete proof. They provide a digital voice recorder to the complainant and lace the currency notes with the Phenolphthalein powder. As it is a colourless powder, it is not visible to the naked eye. The currency number is noted down for later confirmation of the same notes. The complainant is now given instructions about the test and is told different ways to signal the anti-corruption team. These signals include scratching your head, giving a missed call, etc.
The complainant is sent to the place where the exchange of currencies is about to take place and the operation begins. The complainant hands over the money to the alleged culprit, causing Phenolphthalein powder to smudge on the culprit’s fingers and in the place where he has kept the money, generally in his pocket or a storage compartment. Then, the complainant signals the anti-corruption agency, who nab the culprit, after which he is interrogated, and the money in his possession is confiscated. The culprit’s hands are dipped in a beaker which contains an alkaline solution of sodium carbonate. If the solution turns pink, it can be taken as evidence of acceptance of bribe by the culprit. The culprit may be asked to submit his clothes for the purpose of further strengthening the evidence. The report produced by the expert becomes secondary evidence as it supports the tests of the results conducted by the anti-corruption agency.
An easy test for confirming the presence of phenolphthalein powder could be using a pH paper. If the solution falls within a pH range of 8.3 to 10, with pink colour, then the test is positive and there is phenolphthalein in the solution. The other scientific methods through which the presence of phenolphthalein could be detected are thin layer chromatograph, UV Spectroscopy, high performance liquid chromatography, High performance thin layer chromatography.
Glaring Flaws in the Phenolphthalein Test
In numerous judgements, the judiciary has held that a positive phenolphthalein test cannot in itself lead to a conviction, and must be instead treated as corroborative evidence.[2] The Supreme Court has, on a number of occasions, relied on the phenolphthalein test to convict the accused for bribery under the Prevention of Corruption Act, 1988 and under IPC.
- Issues highlighted by the Judiciary
The test has been used by the Anti-Corruption bureau for the past seven decades! However, certain technical issues have been pointed out by the judiciary. In the case of N Vijaya Kumar vs State of Tamil Nadu, the Supreme Court refuted the positive results of a Phenolphthalein test by stating that a gap between the setting up of the trap and the occurrence of the test, even a gap of merely an hour, would invalidate the test. Such a gap would result in suspicion and the culprit could not be proven guilty without reasonable doubt. In another case, it was held that the use of a pen for mixing the powder in the solution would also invalidate a positive phenolphthalein test[3]. Finally, the Supreme Court considered the test to be corroborative evidence because the hands of the accused could be tainted while denial during the handling of the currency notes.
- Issues highlighted by scientists
A chemical flaw of the Phenolphthalein test is that if the test is positive, i.e., the solution results in a pink colour, then the test result is sent to the forensic lab with other relevant evidentiary material to establish it as evidence. However, the pink colour of the solution fades away after a period. Such discoloration depends upon the quantity of the phenolphthalein powder which got mixed into the solution and the strength of the solution of sodium carbonate. This is due to the break down into 2(4- hydroxy benzoyl) benzoic acid and phenol in alkali medium. This becomes an issue during the trial since after a prolonged period, when the positive phenolphthalein solution is presented in the court, it turns colourless, which makes it difficult to confirm it as positive. However, forensic reports produced soon after the testing can conclude it as positive and later be presented in Court.
Altering the formula of phenolphthalein
To maintain the pink colour present in a positively tested phenolphthalein solution for a longer period, the trap party should mix the phenolphthalein powder with hydroquinone before smudging the currency notes. This maximizes the time period for which the solution remains pink. It further reduces the chances of the accused claiming that the pink colour of the solution is due to the cathartic and aperients material, as during the thin layer chromatography test the pink coloured spots can only occur due to the presence of hydroquinone in phenolphthalein.
However, this issue is caused due to human error, or due to a lack of proof, which makes it tough to resolve.
Alternative to phenolphthalein
Further, the phenolphthalein test could be completely replaced by Anthracene powder. It does not fade away like the phenolphthalein powder and gives a natural advantage due to its fluorescence colour. Moreover, there is no need to form a solution as in the case of phenolphthalein test. The powder can be directly examined under U.V. light as it appears as a fluorescent colour under it. Pure anthracene will show as a blur fluorescent colour and impure anthracene will show as a yellow with green fluorescent colour.
[1] Raghbir Singh v. State of Punjab AIR 1976 SC 91
[2] Narayana v. State of Karnataka 2010 SCC 14 453
[3] State Of Rajasthan v. Murlidhar Gaur 2013 CRICC 3 419
Shashank Maheshwari
3rd Year (VI Sem), NMIMS School of Law, Mumbai (Kpmsol)